
Bio-Pharmaceuticals & Complex Generics
We developed complex generic molecules using our innovative platform technologies which are at pre-commercialization stage or preclinical stage,
L-Asparaginase
Peg-Asparaginase
L-Asparaginase and PEG-Asparaginase are enzymes used to treat acute lymphoblastic leukemia and lymphoblastic lymphoma.
Continuous research efforts of Lorven Scientific team, identified Asparaginase producing wild type bacterial strains and further improved L-Asparaginase yields to gram/Lit level.
Lorven L-Asparaginase is highly efficacious and free from traces of BET and bacterial cell wall related impurities.
Lorven research team developed PEG-Asparaginase with high potency can be directly used into final product formulations.
Recombinant Human serum albumin
Human serum albumin (HSA), the most abundant protein in human blood plasma, is essential for maintaining osmotic pressure.
It is produced in the liver, consists of a single polypeptide chain, with 585 amino acids with 17 tyrosil residues and one tryptophan located in position 214.
Liraglutide is a GLP-1 analog used in the management of type 2 diabetes mellitus and prevention of cardiovascular complications associated with diabetes.
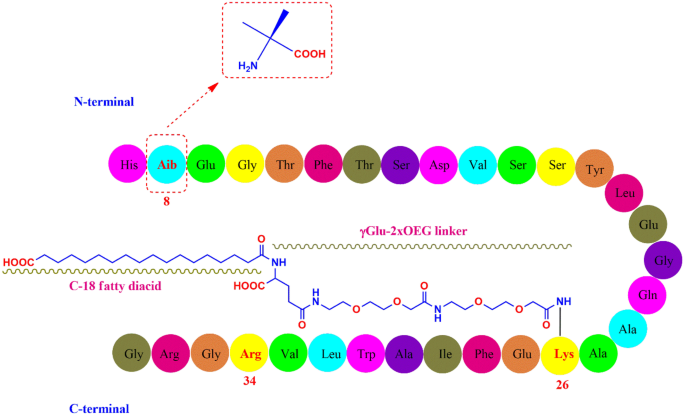
Liraglutide is 97% similar to native human GLP-1, differing primarily by substituting arginine for lysine at position 34.1Liraglutide is made by attaching a C-16 fatty acid (palmitic acid) with a glutamic acid spacer on the remaining lysine residue at position 26 of the peptide precursor.
Liraglutide was developed by Novo Nordisk and approved by FDA on January 25, 2010.
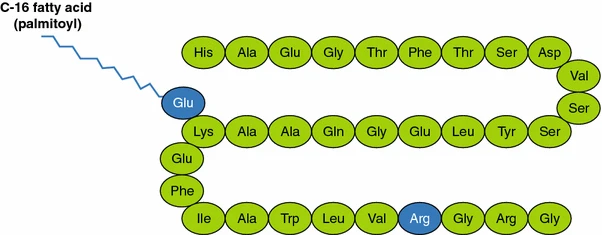
Our engineered Yeast derived Liraglutide is at Preclinical stage.
Semaglutide
Semaglutideis a glucagon-like peptide 1 receptor agonist used to improve glycemic control in type 2 diabetes mellitus
Semaglutide was developed by Novo Nordisk and approved by the FDA for subcutaneous injection in December 2017.
The tablet formulation was approved for oral administration in September 2019. Semaglutide works by binding to and activating the GLP-1 receptor, thereby stimulating insulin secretion and reducing blood glucose
Our engineered yeast- derived semaglutide is at POC stage.
Ranibizumab was initially approved by the FDA in 2006 and by the European Commission (EC) in 2007.
Ranibizumabis a recombinant humanized monoclonal antibody and VEGF-A antagonist used for the management of macular edema after retinal vein occlusion, age-related macular degeneration (wet), and diabetic macular edema.
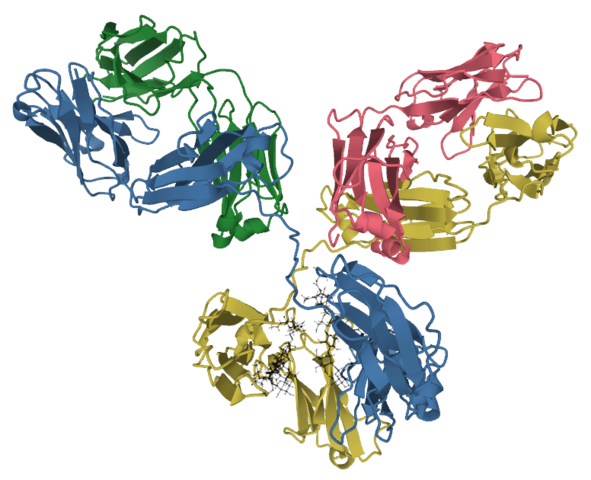
It is marketed under the brand names LUCENTIS and SUSVIMO.
Reach us
Operations
D No 5-62/2, BNR Building, Dulapally Road, Medchal, Malkazgiri, Kompally, Hyderabad, Telangana-500014.
Registered
D No 80-1, Modugulapeta Village, Govindapuram Post, Santhakaviti Mandal, Srikakulam, Andhra Pradesh-532123.
Contact info
Phone Number
+91 77805 68694
info@lorvenbiologics.com
